Integrin Signaling 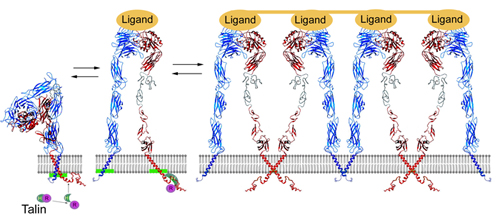
Integrins comprise a large family of cell-surface adhesion receptors that are found in many animal species, ranging from sponges to mammals. Integrins control cell migration and anchorage and, therefore, are crucial for many biological processes including embryogenesis, haemostasis, immune response and maintenance of the tissue integrity. Improperly balanced stickiness of the cells, caused by the integrin mutations or misregulation, contributes to a number of serious human disorders, including rheumatoid arthritis, heart attack, stroke and cancer. Therefore, understanding the mechanism of the integrin function is of both physiological and pathological significance.
Intact integrin receptors are noncovalent heterodimers. The extracellular domains bind variety of ligands, whereas the intracellular cytoplasmic tails anchor to cytoskeletal/ signaling proteins. Integrins are unique in their bi-directional signal transduction capabilities. Although significant progress has been made in the molecular understanding of integrins activation, referred to as "inside-out" signaling, much less is known about early intracellular events following the integrin mediated extracellular matrix engagement, a process termed as "outside-in" signaling. Among the earliest biochemical responses related to outside-in signaling are tyrosine kinases activation and phosphorylation of β integrin cytoplasmic tails. We have investigated and defined how tyrosine phosphorylation affects the structure of β3 integrin cytoplasmic tail and its interactions with several intracellular target proteins.