Nanodiscs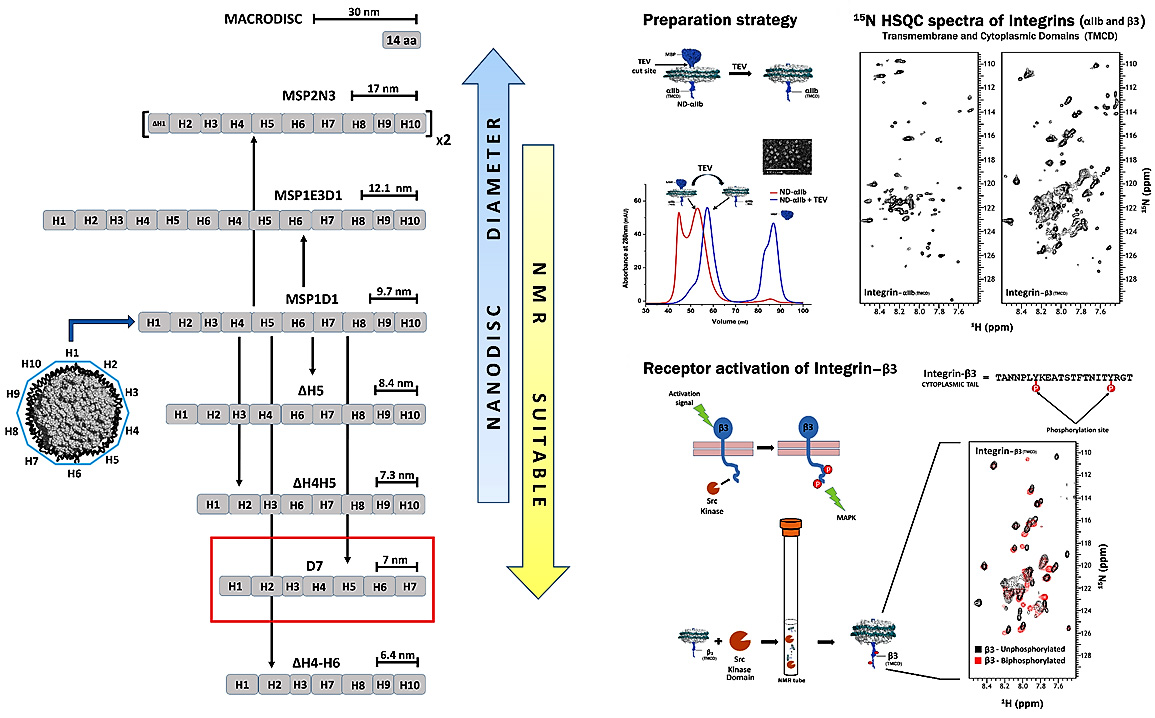
Nanodiscs are discoidal lipoproteins, having a nanoscale phospholipid bilayer held together by an amphipathic helical membrane scaffold protein (MSP) derived from apolipoprotein A-I. The protein belt constrains the dimensions of the bilayer and ensures monodisperse size distributions. The lipid content, which defines the thickness of the bilayer, is varied by application of a variety of lipids including DMPC, POPC, DPPC, E. coli lipid extracts, etc. Due to their solubility, ease of concentration, monodispersity, temperature stability, and compatibility with cell free expression, nanodiscs present a competitive edge over other systems available for studying membrane proteins. We have made nanodiscs more suitable for solution NMR applications by reducing the diameter of the self-assembly complex to its potential limit and also developed an on-column method for a rapid, efficient, single step preparation of nanodiscs incorporated with proteins of interest (2013 paper). As an example, we have successfully incorporated integrin αIIb- and β3-TMCDs into minimal D7 nanodiscs and we collected 15N-TROSY-HSQC spectra of excellent quality for both. The overall number of peaks matches with expected number of amides in both integrin subunits, demonstrating that our D7 discs constitute an excellent membrane system for solution NMR studies of cell surface receptors with single TM domains prone to oligomerization. We have also mixed the 15N-labeled β3 discs with the kinase domain of Src kinase along with ATP molecules in an NMR tube and collected a 1H-15N TROSY HSQC spectrum. By overlaying the un-phosphorylated and bi-phosphorylated spectra, one can clearly see shifts arising due to the phosphorylation of β3 tails in nanodiscs. Thus, we have shown a simple application, free from the need for deuteration, which can be routinely applied with a medium field NMR magnet to study receptors signaling (2017 paper). We are now using this system for different projects to study cell surface receptors in their native lipid bilayer environment.